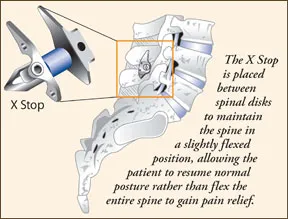
Spinal decompression has been promoted for the relief of lumbar spinal stenosis since the Food and Drug Administration (FDA) approved the cervical disc implant X Stop several years ago. The implant is placed between the spinous processes-the thin projections from the back of the spinal bones to which muscle and ligaments are attached-in the lumbar spine. Its designed to treat patients 50 and older suffering from pain or cramping in the legs secondary to a diagnosis of lumbar spinal stenosis. Two similar implants are now undergoing FDA review, but "functionally, theyre all the same," says Douglas Orr, MD, a staff physician in the Center for Spine Health and the Department of Orthopaedic Surgery at Cleveland Clinic.
To continue reading this article or issue you must be a paid subscriber.
Sign in