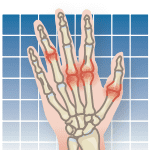
A new medication offers patients with rheumatoid arthritis (RA) an alternative to the injectable drugs commonly used to manage the disease. In November 2012, the U.S. Food and Drug Administration (FDA) approved tofacitinib, the first oral biological response modifier. The drug, marketed as Xeljanz, provides a new way to treat RA if other therapies prove unsuccessful. Its an exciting advance, says M. Elaine Husni, MD, vice chairman of the Department of Rheumatic and Immunologic Diseases and director of the Arthritis and Musculoskeletal Treatment Center at Cleveland Clinic. I say it cautiously because the existing drugs we have are so good.
To continue reading this article or issue you must be a paid subscriber.
Sign in