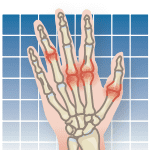
In light of the development of new anti-arthritis drugs, as well as now-proven combinations of medications, the American College of Rheumatology issued updated guidelines in July for the treatment of rheumatoid arthritis (RA). The new recommendations focus on several classes of anti-arthritis drugs, including disease-modifying anti-rheumatic drugs (DMARDs) and newer biologic drugs, called anti-TNF agents. Among the key recommendations: the DMARDs methotrexate or leflunomide are recommended for most RA patients; anti-TNF drugs etanercept, infliximab, or adalimumab along with methotrexate can be used in early RA cases with worsening symptoms; methotrexate, leflunomide, or biologics should not be administered if RA patients have a bacterial infection, shingles, or hepatitis; and anti-TNF agents should not be prescribed for patients with a history of heart failure. Authors of the new guidelines cautioned that their recommendations are based on clinical evidence and expert-panel input and are not intended to limit a physicians clinical judgment or replace individualized medical decisions. The last set of RA guidelines was published in 2002.
To continue reading this article or issue you must be a paid subscriber.
Sign in